A molecule made by working muscles may help exercise work even better—at least in mice. In a new study, middle-aged male mice that ran on wheels while receiving the exercise-linked metabolite L-β-aminoisobutyric acid (L-BAIBA) built stronger slow-twitch muscles, sturdier bones, and carried less fat in their bone marrow than runners without the supplement.
The findings, published in Aging (Albany NY), illuminate how a single exercise-induced metabolite could fine-tune the body’s response to training and aging, and point to intriguing possibilities for future therapies—while underscoring that the evidence so far comes from animals, not people.
What is L-BAIBA, and why it matters
When we move, skeletal muscle releases a host of signaling molecules that ripple across the body. Among them is β-aminoisobutyric acid (BAIBA), a non-protein amino acid produced from valine and thymine metabolism. BAIBA exists in two forms, D- and L-; recent work shows that skeletal muscle secretes predominantly the L- isomer in response to contraction.
Prior studies have linked BAIBA to improved metabolic health, browning of white fat, and protection against disuse-related musculoskeletal decline. The new research asked a next-step question: in healthy, middle-aged animals, does combining routine endurance exercise with L-BAIBA supplementation amplify benefits compared with exercise alone?
Inside the study
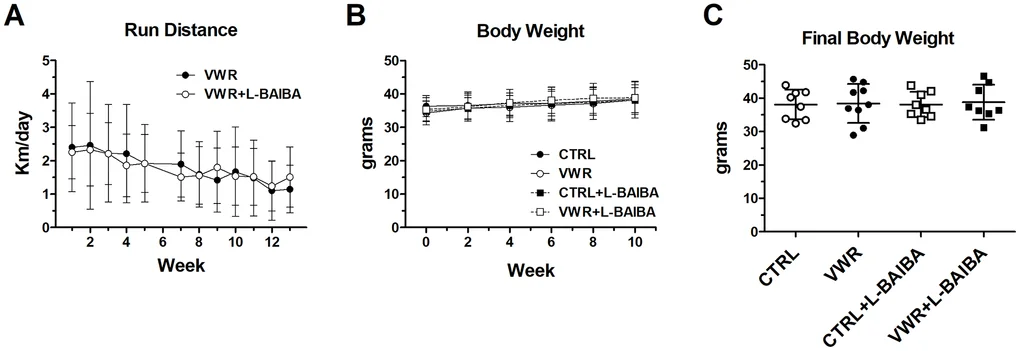
Researchers at the University of Missouri and Indiana University studied 12-month-old male C57BL/6 mice—a model of middle age—and assigned them to four groups for three months: sedentary control; exercise via voluntary wheel running (VWR); L-BAIBA alone (100 mg/kg/day in drinking water); or VWR plus L-BAIBA. Mice ran an average of roughly 1.8 km per day, and running distances and body weights were similar between the VWR and VWR+L-BAIBA groups throughout the study.
At the end of the intervention, the team assessed heart rhythms, isolated muscle size and strength (slow-twitch soleus and fast-twitch extensor digitorum longus, or EDL), bone structure by micro-CT, mechanical properties via three-point bending, and bone marrow adipose tissue.
Muscle: slow-twitch gains, fast-twitch trade-offs
The clearest effects appeared in the soleus, a postural, slow-twitch muscle. Compared with sedentary controls, mice that ran and received L-BAIBA had heavier soleus muscles and significantly greater contractile force—about a 15% increase at maximal stimulation and 27% at submaximal levels. Specific force was unchanged, indicating the strength gains stemmed from more contractile tissue (hypertrophy) rather than intrinsic changes in muscle quality.
Histology reinforced the functional gains. The soleus in the VWR+L-BAIBA group shifted toward a more oxidative profile, with type I fiber proportion rising to 55.7% versus 40.2% in controls, and type IIA fibers falling accordingly. The muscle’s force–frequency curve shifted leftward—consistent with greater calcium sensitivity and slow-fiber content—and the rate of relaxation at submaximal force was faster. Voluntary running alone nudged these trends but generally did not reach statistical significance against controls.
The fast-twitch EDL told a different story. Voluntary running alone improved early fatigue resistance and boosted recovery in response to caffeine (a proxy for sarcoplasmic reticulum calcium release), but those benefits were blunted when L-BAIBA was added. No significant EDL changes in mass, fiber type composition, or specific force were observed across groups. Together, the results suggest L-BAIBA preferentially enhances slow-oxidative muscle adaptation while largely preserving fast-twitch properties.
“The findings of this study indicate a physiological interaction between endurance exercise and L-BAIBA supplementation to enhance soleus muscle mass, strength, type I myofiber content, bone structural and material qualities, and prevent an increase in bone marrow adipose content,” the authors wrote.
Bone: denser network, stiffer material, less marrow fat
Endurance running by itself did not significantly reshape bone in these middle-aged males. L-BAIBA changed that picture. Mice that received L-BAIBA—either with or without running—had bones with a higher modulus of elasticity (stiffer material), alongside a smaller moment of inertia due to a smaller cortical diameter. Micro-CT revealed thicker and better-connected trabeculae in the VWR+L-BAIBA group compared with controls, and lower measures of trabecular pattern factor (a sign of improved connectivity).
Perhaps most striking, bone marrow adipose tissue dropped sharply with the combination of exercise and L-BAIBA—by 63% versus sedentary controls—while running alone did not reduce marrow fat. Because marrow adiposity is tied to bone fragility and systemic metabolic health, the shift hints at broader benefits in energy metabolism and bone remodeling.
Overall, the data suggest L-BAIBA may help maintain trabecular connectivity and material quality during aging, potentially reducing the need for the skeleton to compensate through outward geometric expansion.
Heart and behavior: a subtle signal, not a sprint boost
On the cardiac side, most electrocardiogram parameters were unchanged, including heart rate and heart rate variability. The combination of running and L-BAIBA produced a modest 4% prolongation of the QTc interval compared with controls, a marker of ventricular repolarization time that can lengthen with training in athletes. Heart weight did not differ among groups.
Notably, L-BAIBA did not make mice run farther. Average running distances over the three-month period were virtually identical between the VWR and VWR+L-BAIBA groups, indicating that the musculoskeletal gains stemmed from altered adaptation, not increased training load.
How might it work?
Several well-known pathways could be in play. BAIBA has been shown to activate AMPK and PPAR signaling—pathways that elevate oxidative metabolism and promote a slow-twitch phenotype—while other studies report BAIBA-associated increases in AKT activity, a positive regulator of mTOR and muscle protein synthesis. The authors also note that, by enhancing oxidative capacity and calcium sensitivity, L-BAIBA could lower the stimulation threshold needed to achieve submaximal force, a range more relevant to daily function than all-out maximal contractions.
At the same time, the blunted caffeine-potentiated recovery in fast-twitch EDL after VWR+L-BAIBA hints at metabolic trade-offs. If BAIBA tilts muscle toward fat oxidation, that could disadvantage rapid, glycolysis-heavy bursts during repetitive isometric contractions—aligning with prior findings from PPARδ stimulation studies.
What it means—and what it doesn’t
The study underscores a recurring theme in aging biology: exercise remains the foundation, but targeted molecules may one day help tailor or amplify its benefits. A supplement that nudges muscle toward a slow-oxidative profile while stiffening bone material and trimming marrow fat could aid recovery from disuse, address aspects of osteosarcopenia, or improve resilience in later life.
But translation to humans will require careful testing. These were male mice only, trained with voluntary endurance running (not resistance), and some baseline parameters weren’t measured prior to the intervention. Moreover, the heart signal—a mild QTc increase without hypertrophy—warrants further exploration. The paper also notes emerging roles for the D- enantiomer of BAIBA, raising questions about how the two forms may interact in people.
“L-BAIBA may lower the threshold of active loading required during exercise to promote maintenance of bone during aging,” the authors wrote, arguing that it could help sub-optimal mechanical stimuli nudge the skeleton toward formation.